Abstract
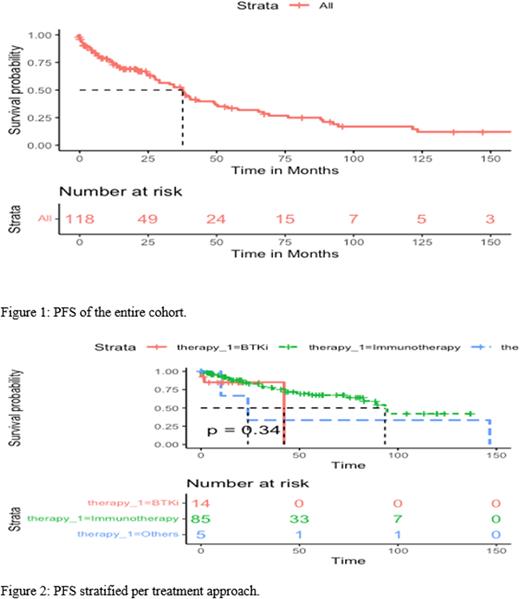
Background: Chronic lymphocytic leukemia (CLL) is relatively uncommon in the Middle East, with a younger age at diagnosis and different clinical characteristics compared to the West. There is little research on CLL in this region. This study aims to evaluate widely used prognostic markers and examine treatment outcomes of CLL patients in the Kuwait Cancer Control Center (KCCC), the only cancer center in Kuwait. Methods: This is a retrospective analysis of unselected 141 CLL patients receiving treatment in the KCCC. Diagnostic and treatment guidelines in our center were in accordance with the International Workshop on CLL criteria. Results: The median age of those patients at diagnosis was 58 years (IQR, 51-65), with 60% of patients under the age of 60. The male to female ratio in this group was 3.3:1. Median absolute lymphocyte count was 51x109/L (IQR, 51-65), and the median hemoglobin and platelet count were 11.8 g/dL (IQR, 10.1-13.8) and 170x109/L (IQR, 129-222). FISH analysis was performed in 118 patients, of which 9% (11) demonstrated del17p. Del13q and trisomy 21 were detected in 31% (36) and 26% (31) patients, respectively. IGHV mutational status was determined in 80 patients, 25% (20) were mutated, and 75% (60) were unmutated. When it comes to treatment, 47% (65) of patients were on FCR regimen, while 11% (15) and 12% (16) were receiving BR and CR, respectively. Approximately 15% (21) of patients in our study were on a BTKi. In 105 patients, the median progression-free survival (PFS) was 44.2 months [95% CI: 36-61.5], with a PFS rate of 48% and 32% at 25 and 50 months, respectively. In 121 patients with no history of progression or treatment in our study cohort, 41.5% and 21% progressed or received treatment at 25 and 50 months, respectively, with a median time to first progression or treatment around 37.6 months [95% CI: 29.1-49.5]. Examining 103 patients with prior history of progression or treatment, 34% and 13% progressed again or required a second course of treatment at 25 and 50 months, respectively, with a median time to second progression or treatment around 65.5 months [95% CI: 40.5-NA]. Those receiving chemoimmunotherapy exhibited a higher median PFS of 93 months [95% CI: 83-NA] compared to those receiving a BTKi with a median PFS of 42 months [95% CI: NA-NA]. However, the difference was not statistically significant (p=0.34). IGHV mutation status was significantly associated with OS (p=0.044) but not PFS (p=0.28). Those with unmutated IGHV had an inferior outcome compared to their mutated counterparts with OS rates at 50 months, around 15% and 25%, respectively, PFS rates were also lower in the unmutated compared to a mutated group, 21% vs. 31% at 50 months, respectively, and PFS medians around 49 [95% CI: 36.4-NA] and 94.5 [95% CI: 94.5-NA], respectively. Multivariate cox regression analysis on OS was not significant (p=0.160). None of the individual treatment regimens were significant predictors of OS compared to the reference (BR), except FCR. Treatment with FCR was associated with a hazard ratio of 0.11 (p = 0.011). Unmutated IGHV status, presence of del17p, and age greater than 60 were all associated with greater hazard ratios, but none were significant predictors of OS. Conclusion: Our CLL treatment group is younger. Unmutated IGHV was associated with a poor prognosis, consistent with the literature. However, treatment with BTKis was not associated with superior outcomes compared to CIT; however, this study is not powered to detect a difference between the two arms. Those receiving FCR had the best survival outcome compared to those receiving other therapies.
Keywords: Chronic Lymphocytic Leukemia, Treatment Outcomes, Kuwait, Middle East
Funding Agency: Kuwait Foundation for the Advancement of Science, Grant number: PR1713MM03
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.